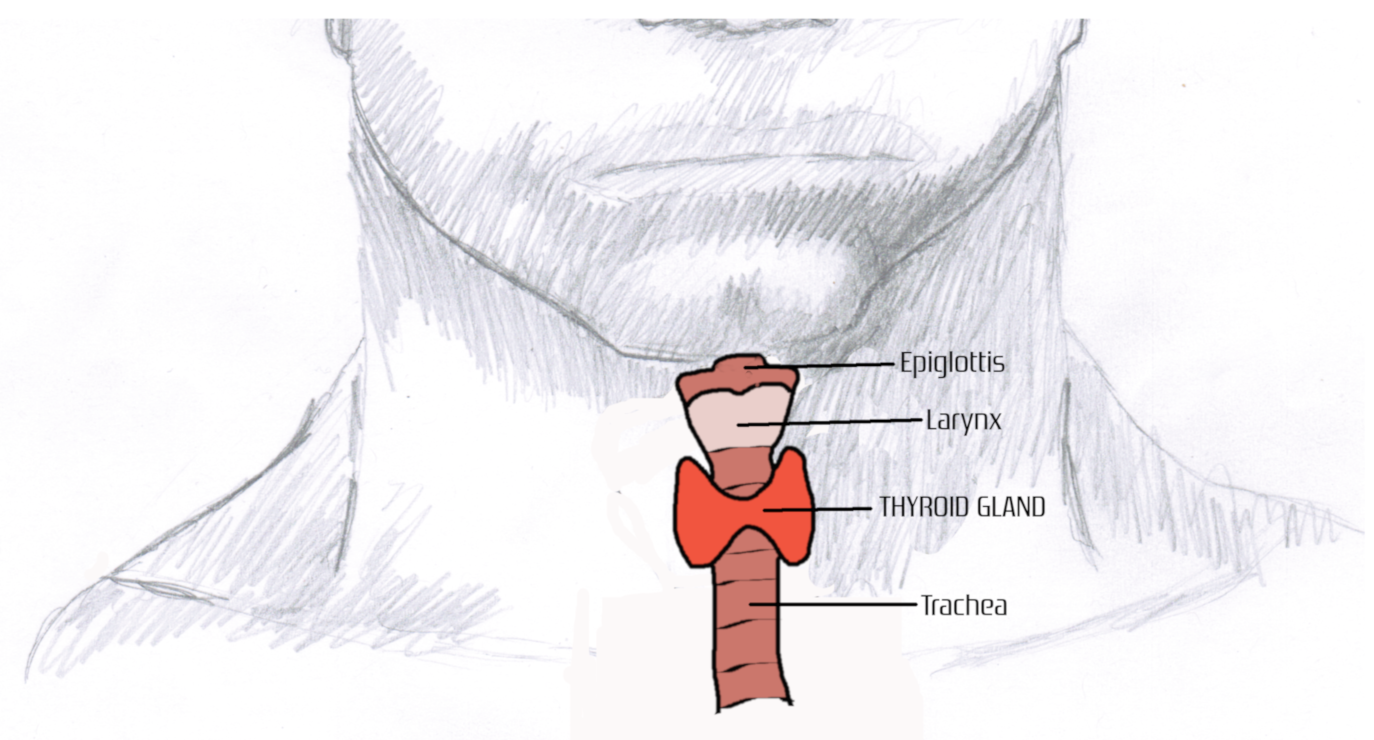
The thyroid is a single gland that contains two main lobes connected by a thin layer of connective tissue. It is located just below the voice box (larynx) and in front of the windpipe (trachea).
Your family doctor likely checks your thyroid gland during medical visits by palpating your neck on either side of your larynx. This is done to feel for abnormalities such as inflammation of the gland (enlargement), nodules, or tumors. If any of these things are suspected, you will need more testing, such as scans or biopsies. However, the most common type of testing is having blood collected to check the actual function of the thyroid.

What is the function of the thyroid?
The thyroid is part of the endocrine system. In a nutshell, this complex system regulates numerous bodily functions through the release of chemical messengers called hormones. The hormones produced by the thyroid are tetraiodothyronine (also known as thyroxine or T4), triiodothyronine (T3), and calcitonin.
There are two primary functions of the thyroid hormones: metabolism/metabolic rate (including fat and carbohydrate metabolism, body temperature, heart rate, protein production, weight management, and energy expenditure) and regulation of calcium. The thyroid also regulates sleep, as excess production of thyroid hormones causes restlessness and insomnia.
Insufficient production of thyroid hormones interferes with ovulation and can be associated with infertility. Increased risk of miscarriage, premature delivery, postpartum hemorrhage, anemia, and pre-eclampsia (significant rise in blood pressure) can also be caused by low thyroid levels. If levels of thyroid hormone are low after delivery, post-partum depression may occur.
Thyroid hormones are also very important for healthy development of the fetus. If the mother’s blood is deficient in thyroid hormones, it may result in severe physical and mental retardation of the fetus. For this reason, it is recommended that thyroid hormone levels are checked for women of childbearing age, even prior to conception.
There are other glands within the endocrine system that work in concert with the thyroid gland to maintain bodily functions. These will be discussed a little later.
Let’s get a little more technical..for those interested
From a histological and biochemical standpoint, the thyroid gland contains follicles that are rich in thyroglobulin (a protein) and specialized parafollicular cells (around the follicles) called C cells that produce the hormone calcitonin.
Our bones serve not only to give our body structural support for our organs and movement of our body, but also as a reservoir for important minerals needed by the body. Calcium is a very important mineral that is stored in bone.
Our bones are continually being built, broken down, and rebuilt. This doesn’t just happen when there is an injury to bone, but regularly, and the result is the release of minerals that are needed to maintain normal internal body stability (homeostasis). Cells that break down bone are called osteoclasts, and cells that build bone are called osteoblasts.
When levels of calcium become too high in the bloodstream, the thyroid releases calcitonin, which suppresses osteoclast activity in bone, prevents calcium absorption by the intestine, and stimulates the kidneys to absorb more calcium for release in the urine. This release of calcitonin is mostly a short-term process and patients with chronically increased calcium levels (hypercalcemia) typically do not show high levels of calcitonin in circulation. High calcitonin levels in the bloodstream can be an indicator of medullary thyroid carcinoma (cancer of the thyroid gland).
Thyroglobulin is a protein comprised of many tyrosine amino acids. Iodine molecules attach to tyrosine, with the help of the enzyme thyroperoxidase (TPO), to form the thyroid hormones.
Diiodotyrosine (DIT) is formed when two iodines are added to tyrosine. When two of these DIT molecules combine (again, through the action of TPO), the hormone tetraiodothyronine (thyroxine or T4) is formed.
The T4 molecule is broken down (catabolized) by removing an iodine molecule to form triiodothyronine (T3). This is done through the action of the enzyme 5′ deiodinase (5DEO), which requires the cofactor selenium. The conversion of T4 to T3 occurs primarily in the liver, but also in the intestines and in circulation.
The majority of T4 and T3 in circulation is bound to proteins. The proteins are albumin and thyroxine-binding globulin (TBG). These protein-bound thyroid hormones are biologically inactive.
Unbound or free T4 and T3 are biologically active and able to induce metabolic activity. For this reason, free T4 (FT4) and free T3 (FT3) are typically the thyroid hormones ordered by clinicians and tested in the clinical laboratory.
Let the concert begin
As mentioned earlier, the thyroid works in concert with other endocrine glands. These glands are the hypothalamus and the pituitary gland.
When there is anticipation for energy needs (example – after a meal), the hypothalamus, a tiny region of the brain that links the nervous system to the endocrine system, produces thyrotropin-releasing hormone (TRH). TRH stimulates the pituitary gland to produce thyroid-stimulating hormone (TSH or thyrotropin).
TSH stimulates the thyroid gland to produce T4. As explained earlier, T4 in circulation can be catabolized to T3. Once these hormones are released and in circulation, metabolic processes can occur.
As long as metabolic processes are needed, positive feedback is supplied to the hypothalamus and pituitary to continue stimulation of the thyroid gland. If excess levels of T4 and T3 build up in circulation, negative feedback is provided to the hypothalamus and pituitary to stop the release of TRH and TSH, thereby preventing the production and release of T4 and conversion to T3.
When these endocrine processes work in synchrony, the concentration of FT4 and FT3 in the bloodstream remain within a narrow and tightly regulated concentration.
The three most commonly ordered blood tests to detect and monitor thyroid disorders are FT3, FT4, and TSH.
Thyroid Gland Disorders
So far, we have discussed:
– thyroid hormone T4 produced by the thyroid gland,
– conversion of T4 to T3 in the liver, intestines, and in circulation,
– Calcitonin released by the thyroid gland (briefly),
– TRH released by the hypothalamus,
– TSH released by the pituitary gland.
Absence, too little production, or too much production of any of these hormones can cause thyroid disorders. Disease can also be caused by damage to the thyroid gland and new tissue growth (neoplasm). So, thyroid disorders can be classified under one of four categories: neoplasms, insufficiency, hyperproduction, and autoimmunity. Below is a table listing the different disorders under each of these categories
| Neoplasms | Insufficiency | Hyperproduction | Autoimmunity |
| Nodules | Hypothyroidism | Hyperthyroidism | Grave’s disease |
| Goiter | Primary hypothyroidism | Primary hyperthyroidism | Hashimoto’s thyroiditis |
| Thyroid cancer | Secondary hypothyroidism | Secondary hyperthyroidism | Other autoimmune disorders |
Thyroid neoplasms will not be discussed in detail. However, let’s take a deeper look at insufficiency, hyperproduction, and autoimmunity.
Hypothyroidism (insufficiency)
An estimated 15% of the population suffers from underactive thyroid disorder or hypothyroidism. This prevalent disorder predominately affects females, often over age 60. Other at-risk populations for hypothyroidism are those with:
– Family history
– Other autoimmune disorders
– Type I diabetes
– Arthritis
– Pregnancy (within 6 months)
– Previous neck or chest radiation treatment
– Previous neck or throat surgery
– Deficient iodine levels in the living environment
Decreased levels of thyroid hormones cause decreased and insufficient metabolism, which leads to obesity and high blood pressure. If untreated, cognitive impairment and depression may arise.
| SYMPTOMS | CLINICAL FEATURES |
|---|---|
| Fatigue | Obesity |
| Sensitivity to cold | Depression |
| Puffy face | High blood pressure |
| Dry skin | Joint pain |
| Constipation | Infertility |
| Unexplained weight gain | Heart disease |
| Hoarseness | Cognitive impairment |
| Joint pain and stiffness | |
| Thinning hair | |
| Elevated cholesterol | |
| Heavy menstrual periods | |
| Muscle weakness | |
| Swollen neck glands | |
| Impaired memory |
Primary hypothyroidism correlates to defects or disease of the thyroid gland itself. This can include insufficient components to make hormones, blocked or defective TSH receptors, damaged hormone synthesis processing, or autoimmune interference.
Secondary hypothyroidism relates to insufficient stimulation of the thyroid, likely due to inadequate production of TRH from the hypothalamus and/or TSH from the pituitary gland.
As far as laboratory findings, primary hypothyroidism is diagnosed when elevated levels of TSH along with decreased levels of FT4 and decreased or normal levels of FT3 are seen. A diagnosis of secondary hypothyroidism is made when TSH levels are low and both FT3 and FT4 levels are low or normal.
Sometimes, before the onset of true hypothyroidism, levels of TSH may be slightly elevated with normal levels of FT3 and FT4. This is considered subclinical hypothyroidism, which may progress to true hypothyroidism.
Below is a recap of laboratory findings for hypothyroidism.
| TSH | FT3 | FT4 | Interpretation |
| ↑ | ↓ or normal | ↓ | Primary hypothyroidism |
| ↓ | ↓ or normal | ↓ or normal | Secondary hypothyroidism |
| ↑ | Normal | Normal | Subclinical hypothyroidism |
Hyperthyroidism (hyperproduction)
Hyperthyroidism is far less prevalent than hypothyroidism. However, similar to hypothyroidism, females are more at risk than males by a ratio of 5:1. Other risk factors are individuals over the age of 60 and those with other autoimmune disorders.
| SYMPTOMS | CLINICAL FEATURES |
|---|---|
| Weight loss | Bulging eyes – ophthalmopathy or exophthalmos |
| Increased appetite | Heart problems |
| Anxiety | Osteoporosis |
| Irritability | Red, swollen skin |
| Shakiness | Swelling at base of neck |
| Sweating | Feverish |
| Heart palpitations | |
| Insomnia | |
| Eye pain | |
| Fine, brittle hair | |
| Heat intolerance | |
| Thinning skin | |
| Light sensitivity | |
| Nervousness |
Primary hyperthyroidism occurs when conditions directly related to the thyroid gland create toxic levels of thyroid hormone. It is sometimes called thyrotoxicosis. It is caused by thyroiditis (inflammation of the thyroid gland), thyroid nodules, thyroid adenoma, or thyroid autoimmune disease (Grave’s disease).
Secondary hyperthyroidism occurs when the thyroid gland is over-stimulated, either by overproduction of TRH from the hypothalamus (rarely) or increased production of TSH from the pituitary gland. It can also be caused by carcinoma or TRH- and TSH-secreting tumors.
As far as laboratory findings, primary hyperthyroidism is diagnosed when decreased levels of TSH along with increased (or normal) levels of FT4 and increased (or normal) levels of FT3 are seen. Secondary hypothyroidism is suspected when there are increased levels of TSH and high (or normal) levels of FT3 and FT4.
| TSH | FT3 | FT4 | Interpretation |
| ↓ | ↑ or normal | ↑ or normal | Primary hyperthyroidism |
| ↑ | ↑ | ↑ or normal | Secondary hyperthyroidism |
| ↓ | Normal | Normal | Subclinical hyperthyroidism |
Autoimmunity
There are primarily two thyroid-related autoimmune diseases: Grave’s disease and Hashimoto’s thyroiditis. Autoimmune disorders occur when your body thinks that normally functioning tissues, or the products they produce, are foreign and attacks them. This occurs by the production of autoantibodies that attack thyroid tissue receptors or inhibit thyroid function regulation.
As with other thyroid disorders, thyroid-related autoimmune diseases are more prevalent in females and have been reported to affect as high as 25% of adult women.
The most common thyroid autoantibodies analyzed in the clinical laboratory are:
– Thyrotropin receptor antibody (TRAb)
– – – also known as thyroid-stimulating immunoglobulins, TSI
– Thyroperoxidase antibody (TPOAb)
– Thyroglobulin antibody (TgAb)
– Thyroxine-binding globulin antibody
Let’s take a look at the first three of these autoantibodies in more detail.
TRAbs (or TSIs) are antibodies that bind to TSH receptors on the thyroid gland and mimic the action of TSH. Since TSH is what stimulates the thyroid gland to produce thyroid hormones, and these antibodies are mimicking TSH, this causes excess secretion T4 and T3. If you recall, high levels of T3 and T4 (with low levels of TSH) indicate hyperthyroidism. Grave’s disease the autoimmune thyroid disease that manifests as hyperthyroidism. So, a patient with hyperthyroidism (high T3 and T4, low TSH) and an abnormally high level of TSI has Grave’s disease.
TPOAbs attack thyroperoxidase (TPO). As mentioned earlier, TPO is an enzyme that is responsible for adding iodine to the tyrosine in thyroglobulin, thus enabling the formation of T4 and conversion of T4 to T3.
Without TPO, you cannot form thyroid hormones T3 and T4. When there are decreased levels of T3 and T4, the pituitary gland releases more TSH to stimulate the thyroid gland to release thyroid hormones. However, no matter how much TSH is being produced, since T3 and T4 cannot be formed, nothing is produced. Therefore, you would see increased levels of TSH with decreased levels of T4 and T3, which indicates hypothyroidism. The additional thing you would see is high levels of TPOAb. These laboratory findings are indicative of Hashimoto’s thyroiditis.
TgAbs attack thyroglobulin, which is the protein present in thyroid follicles needed to form both T4 and T3. The presence of TgAb alone is not indicative of a specific thyroid autoimmune disease. These antibodies are often found together with other anti-thyroid antibodies. However, thyroglobulin levels can be used as tumor markers for thyroid carcinoma (cancer), and determining TgAb can be essential when monitoring thyroglobulin for this purpose.
In Summary
A lot of information was just presented in order to explain the function of the thyroid and various thyroid diseases. Below is a list of the key components to help summarize everything you just read.
- The thyroid gland is located just below the larynx and in front of the trachea. When your doctor palpates the front of your neck, he/she is feeling for enlargement of the thyroid gland or any palpable nodules/tumors.
- Thyroid diseases include hypothyroidism (insufficiency or underactive thyroid), hyperthyroidism (hyperproduction or overactive thyroid), thyroid cancer, Grave’s disease (autoimmune hyperthyroidism), and Hashimoto’s thyroiditis (autoimmune hypothyroidism).
- Hormones produced by the thyroid are T4 (tetraiodothyronine or thyroxine), T3 (triiodothyronine), and calcitonin.
- The hypothalamus is a tiny region in the brain that releases TRH (thyroid-releasing hormone) when levels of T3 and T4 are decreased.
- In response to TRH released by the hypothalamus, the pituitary gland releases TSH (thyroid-stimulating hormone), which stimulates the thyroid to make more T3 and T4.
- The majority of T3 and T4 in circulation is bound to proteins and are biologically inactive. Unbound or free T3 (FT3) and free T4 (FT4) are biologically active and able to induce metabolic activity.
- The most common tests ordered on bloodwork to detect thyroid disorders are TSH, FT4, and FT3.
- TSH is often the first laboratory test looked at by clinicians, with decreased levels typically indicating hyperthyroidism and increased levels typically indicating hypothyroidism. This may seem confusing, but remember TSH is produced to stimulate the thyroid. If the thyroid is not working properly or sluggish (hypothyroidism), it will ignore the TSH, and levels of TSH will build up in the bloodstream.
- Autoantibodies formed in autoimmune thyroid disease include thyroid-stimulating immunoglobulins (TSI), thyroperoxidase antibody (TPOAb), thyroglobulin antibody (TgAb), and thyroxine-binding globulin antibody.
- Hypothyroidism (↑TSH, ↓FT4, ↓or normal FT3) + ↑TPOAb = Hashimoto’s thyroiditis
- Hyperthyroidism (↓TSH, ↑FT4, ↑or normal FT3) + ↑TSI = Graves disease