Most of us would think 99.9% accuracy sounds pretty good on paper. But in the lab, that can translate to 1 error per thousand tests. Depending on your workplace, you might run anywhere from several hundred to several thousand tests in one shift! Would you be satisfied with your work if there was an error in it every single day? What are the implications for the patients? Are the providers going to trust our work?
Six Sigma was an ideology developed in the manufacturing industry to improve quality and reduce errors and their associated costs, thereby providing more value to potential customers. The term “Six Sigma” refers to the statistical distribution of a process and how often errors occur. For a process to be considered Six Sigma, the incidence of errors (or defects) is 3.4 per million opportunities.
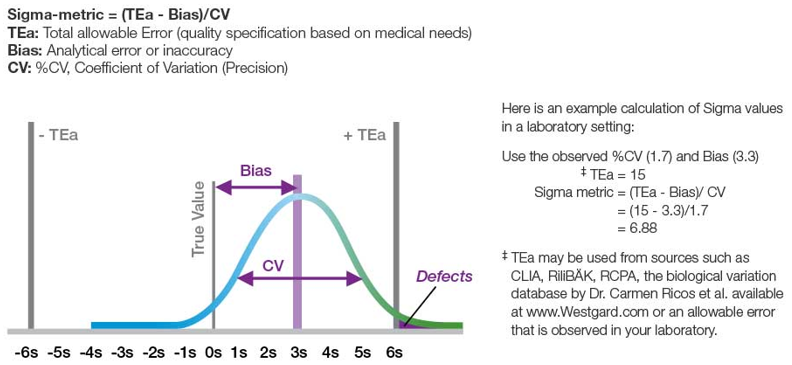
In the context of the lab, sources of error are typically defined as pre-analytical, analytical, and post-analytical. When selecting a project, it is important to consider its impact on the business goals of the organization and how resource-intensive the project will be. It might sound really nice to throw all of your current analyzers out on the street and install some brand new ones with tons of great features. However, how would your lab operate during that transition? And what percentage of your quality shortcomings are from the analytical process?
Let’s say that MLSH Laboratories is a standalone lab that performs routine testing for a variety of primary care providers. The specimens arrive with many different types of requisitions, and there have been calls from the offices stating problems with incorrect testing, missing testing, not receiving results, or reports with incorrect patient information. As part of the project team for MLSH Laboratories, you and your team decide to investigate these issues using the Six Sigma principles.
Phases of Six Sigma
First is the Define Phase. This is where our project team gets together to describe what we plan to do. Expectations from customers are to provide 100% accurate results, and we need to determine where in the process are falling short.
Next is the Measure Phase. We will observe our current project process and decide how to collect data from that process. This data will help pinpoint the cause of the errors and how frequently they are occurring. This will establish our baseline so we can determine if our project is successful. For our project, we will examine any order for which we receive a complaint and a few random samples per office per day.
The next step is the Analyze Phase, where we analyze the data we collected. This should help us determine the root cause of our errors. From the random sampling and complaint calls, we estimated that our frequency of errors was around 3%. We looked at our data to see if we could spot any trends. Mondays and Thursdays tend to be our busiest days; are people rushing through their work and making mistakes? We found no statistical difference in errors depending on the day of the week. However, we did notice a change in the frequency of errors depending on where the specimen came from. So, we pulled a day’s worth of orders from the two offices that had the most discrepancies. Most of the requisitions from those offices looked like this:

We are now ready to move to the Improve Phase. With hand-written orders, it is easy to see why clerical errors were being made that affected our results. The question is, how do we solve this problem? It would be nice to give the providers access to our LIS so they could directly input the orders, but that is a very costly process that we would have to repeat with every new client. We could ask the providers to write more legibly…good luck with that! We could reject samples if any part of the order is unclear, but that would create a lot of extra work for us and a lot of dissatisfaction from our customers. After some brainstorming, our team decides to create a standardized requisition form. It will include our contact information, a defined area for patient information, fax numbers, and our entire test menu with boxes to check. We send these requisitions out to our clients, with an e-mail kindly requesting that all orders sent to our lab be completed on this new form.
Finally, we move to the Control Phase. In this phase, we observe the improved process over time to make sure the changes stay in place. We let staff know that if a sample comes with a hand-written order that is difficult to read, they are well within their rights to request a new order or reject the sample. We make sure any new client gets the standardized requisition. We continue to observe our lab for errors. And we find that our unpleasant phone calls have all but vanished and our random sampling is free of clerical errors!
In Summary
Of course, this is just a very basic implementation of Six Sigma. Six Sigma can be applied in other areas of the lab, such as turnaround times and safety, to name a few. Many other issues will not have such simple resolutions or may involve a detailed risk assessment. But as our clinical laboratories continue to strive to meet the ever-increasing demands of accuracy and timeliness, with an ever-tightening budget and staffing pool, it is important to always be looking for ways to improve quality and efficiency.