In the clinical laboratory, we think of lysis as the destruction or break down of a cell, as in hemolysis. However, lysis also means that something is degraded or broken apart into smaller pieces. Such is the case with fibrinolysis.
Once an injured blood vessel is healed, the fibrin clot that was formed as a result of primary and secondary hemostasis is lysed, restoring normal blood flow and regeneration of a healthy blood vessel.
Fibrinolysis occurs as part of the natural hemostatic cycle. It can also be triggered through medical intervention by administering a fibrinolytic agent, or by the presence of certain medical disorders.
Why is fibrinolysis important?
The coagulation cascade (discussed in the Hemostasis – Part 1 blog), must be kept in check so as not to cause widespread thrombosis. Without fibrinolysis, blood clots would continue to form and grow. Eventually, they would press on other structures and block blood flow to tissues supplied by the blood vessel.
There are two types of fibrinolysis.
Primary fibrinolysis is a natural, physiological process that either 1) triggers the breakdown or destruction of the fibrin or fibrinogen, or 2) prevents thrombin from causing the conversion of fibrinogen to fibrin.
Secondary fibrinolysis is the breakdown of blood clots due to a medical condition or through the administration of a fibrinolytic agent.
Components and Process of Primary Fibrinolysis
So far, we have focused on the formation of a clot (thrombus) and how a blood vessel’s damaged endothelial cells can provide positive feedback to both platelets and the coagulation cascade to continue the coagulation process.
As discussed in our Hemostasis – Part 1 blog, thrombin is a very important factor in the coagulation process, acting as a procoagulant. However, thrombin also plays a big part in the down-regulation of fibrin production through negative feedback.
As mentioned above, one of the ways primary fibrinolysis occurs is through anticoagulation, by preventing thrombin from converting fibrinogen to fibrin. This is done in one of two manners.
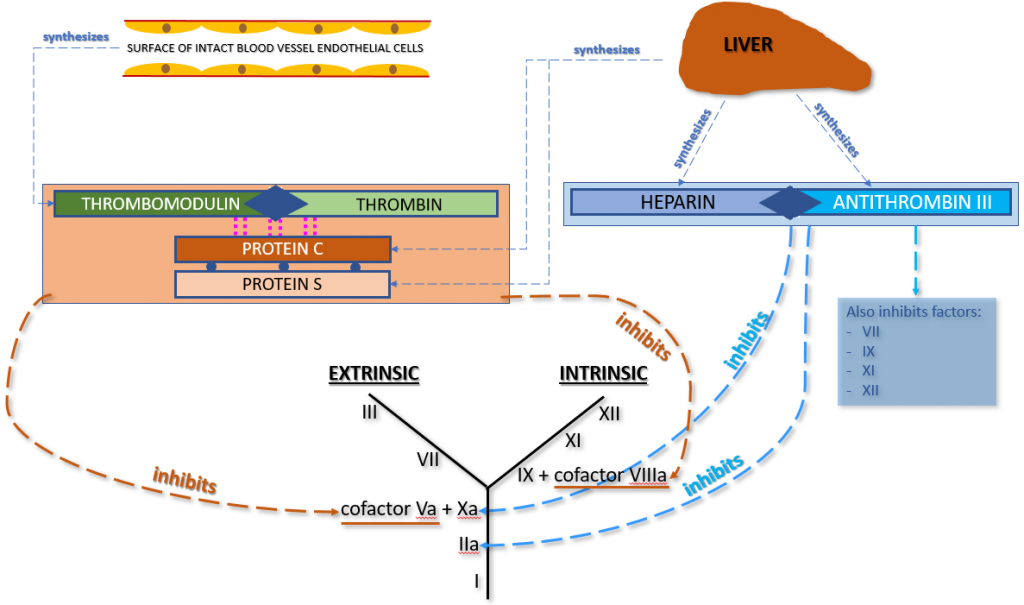
The first manner in which our innate anticoagulation system prevents thrombin from converting fibrinogen to fibrin involves thrombomodulin, thrombin, Protein C and Protein S. Thrombomodulin (present on intact vascular endothelial cells) binds up any unused thrombin that is lingering around the site of blood vessel injury. This thrombomodulin-thrombin complex triggers the activation of Protein C and Protein S (both produced in the liver and circulating in plasma), forming a new complex that contains Thrombomodulin-Thrombin + activated Protein C + activated Protein S. This new complex inhibits Factors Va (Common Pathway) and VIIIa (Intrinsic Pathway) in the coagulation cascade, preventing the conversion of fibrinogen to fibrin. Therefore, the clotting process is halted.
The second manner in which our innate anticoagulation system prevents thrombin from converting fibrinogen to fibrin involves the combination of anti-thrombin III (AT3 or AT) + innate heparin (both produced in the liver). These two components combine and inhibit both Factor IIa and Factor Xa in the coagulation cascade. Since both of these coagulation factors are in the Common Pathway of the coagulation cascade, their inhibition prevents the conversion of fibrinogen to fibrin. Therefore, the clotting process is halted. Anti-thrombin III also inhibits Factors VII, IX, XI, and XII.
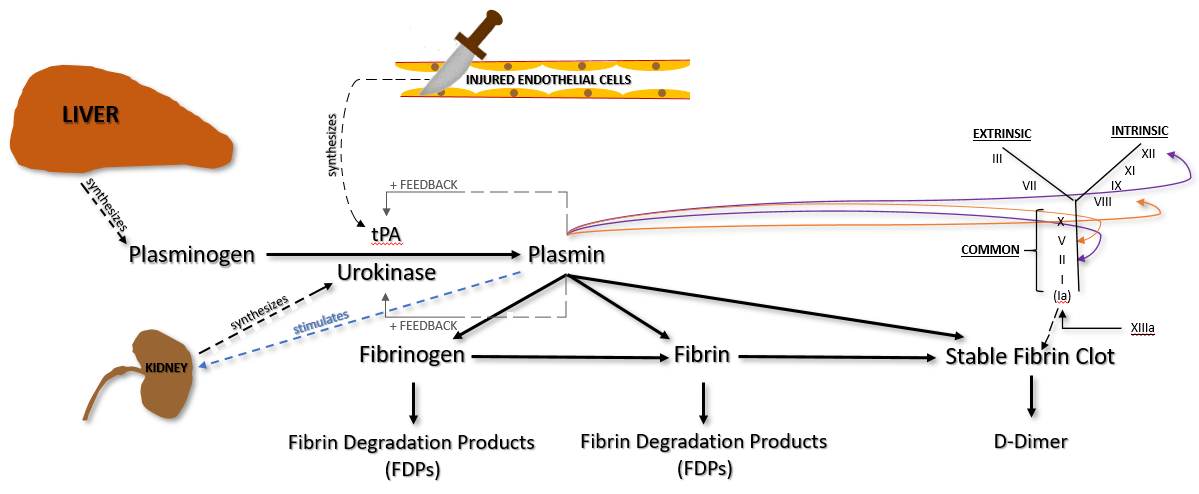
The other way that primary fibrinolysis occurs is by the destruction of fibrinogen and/or fibrin (both insoluble and soluble fibrin). The two important factors involved in this process of fibrinolysis are plasminogen and tissue plasminogen activator (tPA).
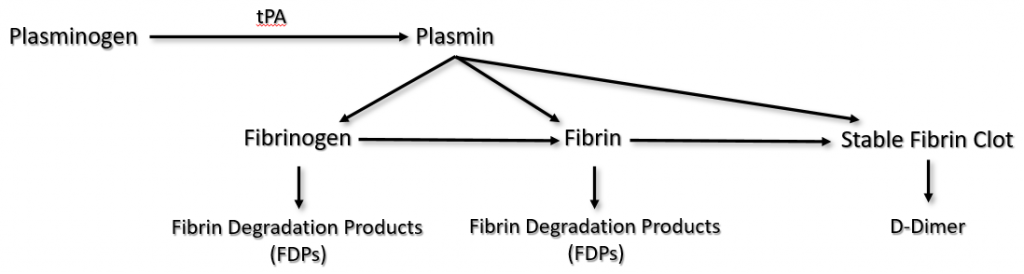
Plasminogen is produced in the liver and circulates in plasma. It becomes incorporated into the fibrin clot as it is being formed.
Injured endothelial cells release tPA, which catalyzes the conversion of plasminogen to plasmin. Plasmin degrades fibrinogen and soluble fibrin into fibrin degradation products (FDPs), sometimes called split products. Plasmin can also degrade insoluble fibrin (the stable fibrin clot) into a protein fragment known as D-dimer.
Plasmin also does the following.
– Provides positive feedback for continued production/release of tPA and urokinase
– Digests Factors V and VIII, both cofactors (inhibits coagulation cascade)
– Digests Factor II (prothrombin) and XII (inhibits coagulation cascade)
It is also important to mention the role of Thrombin in the fibrinolytic process. Thrombin provides 1) negative feedback of the coagulation cascade by stimulating the production of ATIII, 2) negative feedback of fibrinolysis by stopping plasminogen, and 3) down-regulation of fibrinolysis by preventing the formation of the thrombomodulin-thrombin complex.
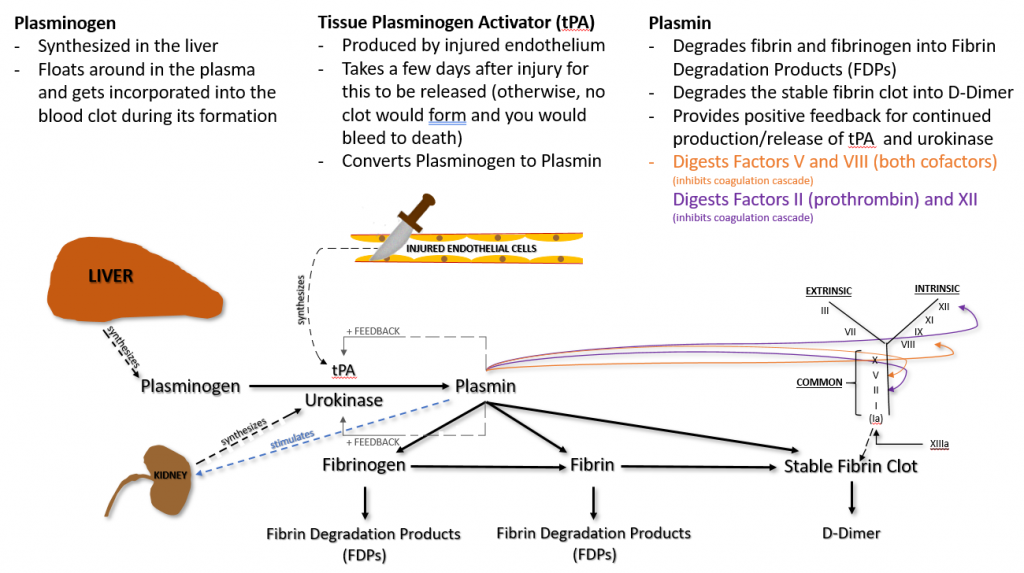
It is important to understand that, while tPA is released from damaged endothelial cells, it is not released until a few days after injury. Otherwise, plasmin would be immediately available and a clot would never form.
Secondary Fibrinolysis
As mentioned earlier, secondary fibrinolysis is the breakdown of blood clots due to a medical condition or through the administration of a fibrinolytic agent.
Some of the medical conditions or activities that can cause secondary fibrinolysis are:
– Trauma,
– Sepsis,
– Surgical procedures in tPA-rich organs (e.g., prostate),
– Peripartum complications,
– Prostate carcinoma,
– Chronic liver disease (cirrhosis), and
– Disseminated Intravascular Coagulation (DIC).
Fibrinolytic agents include:
Heparin – While we have naturally occurring heparin in our bodies, heparin can also be used as a medical fibrinolytic agent. Heparin greatly augments the action of Antithrombin III, thereby preventing the formation of fibrin. When being used as an actual fibrinolytic agent, heparin is administered to inpatients as unfractionated heparin via continuous IV infusion and closely monitored through frequent blood testing. Other forms of heparin therapy, as well as specific blood tests for monitoring therapeutic regimens, will be discussed in a future Hemostasis – Part 3 blog.
Synthetic tPA is used to rapidly break down clots (thrombi) that have formed as a result of an ischemic stroke, ST-Elevation Myocardial Infarction (STEMI), or a large pulmonary embolism.
Streptokinase is a protein produced by Group A Streptococcus (Streptococcus pyogenes). It is used as a thrombolytic agent to disintegrate blood clots that have formed in blood vessels. It is most often used immediately after initial symptoms of a heart attack to improve patient survival. Streptokinase acts by catalyzing the conversion of plasminogen to plasmin. It may also be used to treat blood clots in the legs (deep venous thrombosis or DVT) and in the lungs (pulmonary embolism).
There are other therapeutic agents that are used to prevent the formation of blood clots. These are usually prescribed to patients that have a history of blood clots, or before and/or after certain types of surgical procedures (e.g., artificial joint replacement). These will be discussed in Hemostasis – Part 3 that will be posted sometime in October 2020.
Excellent synopsis of the antifibrinolytic stage of hemostasis. Well written and easy to follow. Will
Certainly use a resource for future teaching. Thank you!
Thank you SO much for your support and kind words. Please feel free to share our site for any of your future teachings; much appreciated.