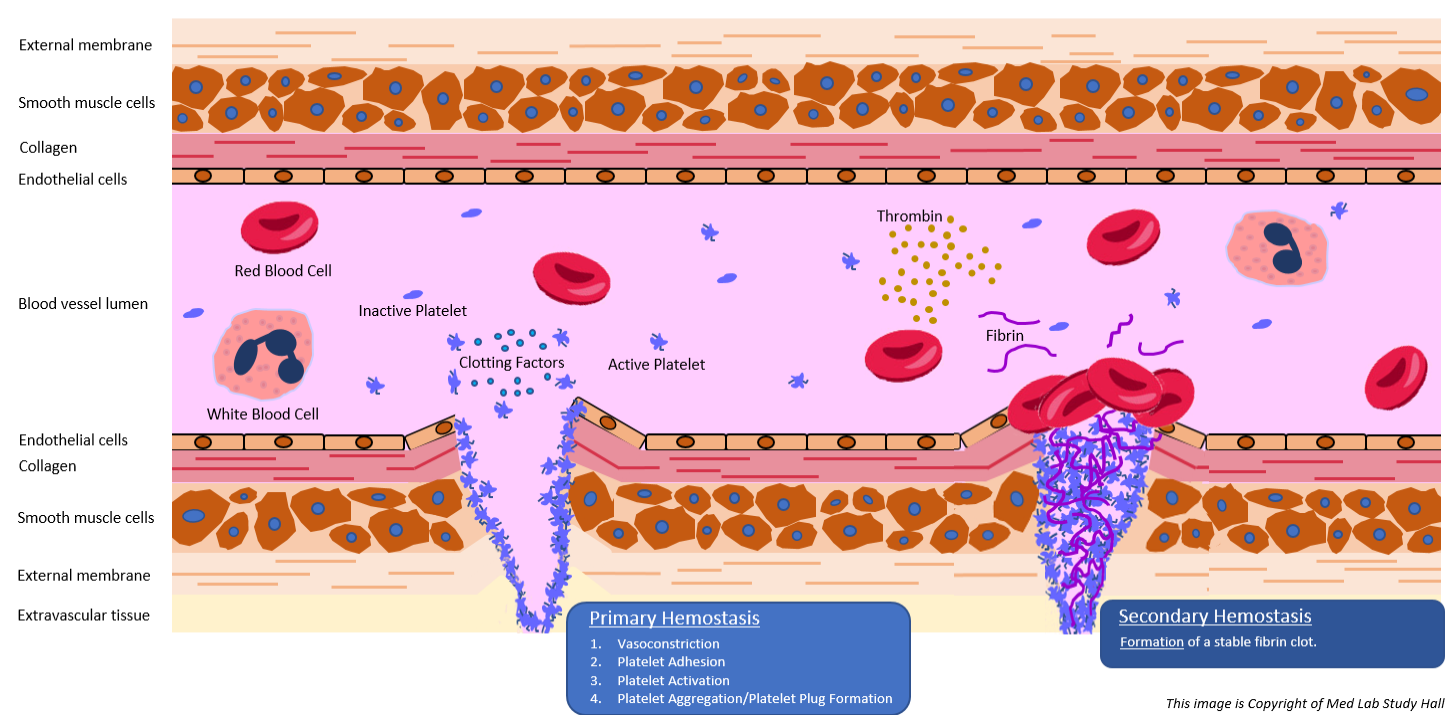
Hemostasis is the process that occurs to stop bleeding at the site of an injury. For most people, this may seem like a simple process; hold pressure on the injury for a few minutes and BOOM…no more bleeding. However, a rather complex series of events take place, involving both cellular components and plasma proteins/enzymes, to stop bleeding at the site of injury while maintaining normal blood flow to the rest of the body.
It is important to understand that, in uninjured, intact blood vessels, our vascular endothelium contains components/mechanisms (natural anticoagulants) that keep our blood in a liquid state. It is upon blood vessel injury that the physiological process of hemostasis kicks in to repair the injured vessel.
This blog is the first in a three-part series on hemostasis and will discuss primary and secondary hemostasis.
Components of Hemostasis
The actual mechanism of hemostasis can be divided into two main components.
- Primary hemostasis – platelet aggregation at the site of injury to form a temporary platelet plug. There are four phases involved in primary hemostasis: vasoconstriction, platelet adhesion, platelet activation, and platelet aggregation.
- Secondary hemostasis – formation of the final, stable fibrin clot, which is triggered by the complex series of events known as the proteolytic coagulation cascade.
Primary and secondary hemostasis occurs simultaneously and their complex mechanisms are interlaced. Let’s take a deeper look at the mechanisms and phases involved in hemostasis by examining what happens from the moment of vessel injury.
Primary Hemostasis
Primary hemostasis involves platelets, which are cellular fragments present in our circulating blood that rush to the site of blood vessel injury to begin plugging the damaged site. Below is explanation of the four stages involved in this process.
>> Vasoconstriction
Injury to a blood vessel causes vasospasm, which stimulates vasoconstriction of that vessel. Damaged endothelial cells in the injured blood vessel expose sub-endothelial collagen and something known as von Willebrand factor (vWF).
>> Platelet Adhesion
As blood flows through the injured vessel, vWF helps platelets become “sticky” and adhere to the vessel wall at the site of injury.
>> Platelet Activation
Once adhered to the site of injury, platelets are activated by thrombin in two manners. First, activated platelets increase their surface area by irreversibly morphing to multi-pseudopod cell fragments. Second, they begin to secrete their cytoplasmic granules. Two of the components released in these granules are Thromboxane A2 (TxA2) and adenosine diphosphate (ADP), both of which help call in more platelets to help plug the injured vessel.
>> Platelet Aggregation/Platelet Plug Formation
Activated platelets form a connection with circulating fibrinogen, which causes platelets at the site of injury to interconnect and form a weak platelet plug.
Secondary Hemostasis
The formation of a stable fibrin clot is the end result of secondary hemostasis. This is achieved by a rather complex series of steps from two intertwined pathways (intrinsic and extrinsic pathways) that involve components known as clotting factors.
There are 12 clotting factors. These factors are commonly referred to by Roman numerals one through five (I – V) and seven through thirteen (VII – XIII), but each also has a specific name (listed later in this blog). It was originally thought there were 13 unique clotting factors. However, as science and technology progressed, Factor VI was actually found to be the activated form of Factor V (Factor Va).
Most of the clotting factors are synthesized in hepatocytes in the liver (Factors I, II, V, VII, IX, X, XI, and XIII). Factors III and VIII originate from endothelial cells, and Factor IV (calcium ions) circulates freely in plasma.
Let’s take a look at the specifics of the intrinsic and extrinsic pathways.
>> Intrinsic Pathway
- Damaged vascular endothelium exposes collagen, which causes Factor XII (Hageman Factor) to be converted to activated Factor XIIa.
- Factor XIIa converts Factor XI (Plasma Thromboplastin Antecedent or PTA) to Factor XIa.
- Factor XIa converts Factor IX (Christmas Factor) to Factor IXa.
- Factor IXa plus Factor VIIIa (which is converted from Factor VIII – Antihemophilic Factor), causes the conversion of Factor X (Stuart-Prower Factor) to activated Factor Xa. Calcium ions (Ca2+) are also needed for this process.
- Factor Xa plus activated Factor Va (which is converted from Factor V – Labile Factor) causes the conversion of Prothrombin (Factor II) to Thrombin (Factor IIa). Calcium ions (Ca2+) are also needed for this process.
- Factor IIa (Thrombin) causes the conversion of Factor I (Fibrinogen) to Factor Ia (Fibrin) and also causes the conversion of Factor XIII (Fibrin Stabilizing Factor) to Factor XIIIa. Calcium ions (Ca2+) are also needed for both of these processes.
- Factor Ia (Fibrin) is acted on by Factor XIIIa, which causes Fibrin to form a mesh that stabilizes the platelet plug into a final, stable fibrin clot.
The overall function of the Intrinsic Pathway is measured in the clinical laboratory with the aPTT blood test (discussed in more detail in a future blog).
>> Extrinsic Pathway
- Damaged tissue releases Tissue Factor (TF or Factor III) and also causes Factor VII (Stable Factor) to be converted to activated Factor VIIa.
- The combination of TF and Factor VIIa causes the conversion of Factor X (Stuart-Prower Factor) to activated Factor Xa. Calcium ions (Ca2+) are also needed for this process.
- Factor Xa plus activated Factor Va (which is converted from Factor V – Labile Factor) causes the conversion of Prothrombin (Factor II) to Thrombin (Factor IIa). Calcium ions (Ca2+) are also needed for this process.
- Factor IIa (Thrombin) causes the conversion of Factor I (Fibrinogen) to Factor Ia (Fibrin) and also causes the conversion of Factor XIII (Fibrin Stabilizing Factor) to Factor XIIIa. Calcium ions (Ca2+) are also needed for both of these processes.
- Factor Ia (Fibrin) is acted on by Factor XIIIa, which causes Fibrin to form a mesh that stabilizes the platelet plug into a final, stable fibrin clot.
The overall function of the Extrinsic Pathway is measured in the clinical laboratory with the PT blood test (discussed in more detail in a future blog).
>> Common Pathway
Take notice that steps 5-7 of the Intrinsic Pathway and steps 3-5 of the Extrinsic Pathway are identical. This series of events is referred to as the Common Pathway. Also, notice that the combination of Factor III (Tissue Factor) and Factor VIIa in the extrinsic pathway can cause the conversion of Factor XI in the intrinsic pathway to Factor XIa. This is sometimes referred to as the Alternate Pathway.
The image below summarizes the complex coagulation cascade involved in secondary hemostasis. It comprises the extrinsic pathway, the intrinsic pathway, the alternate pathway, and the common pathway.
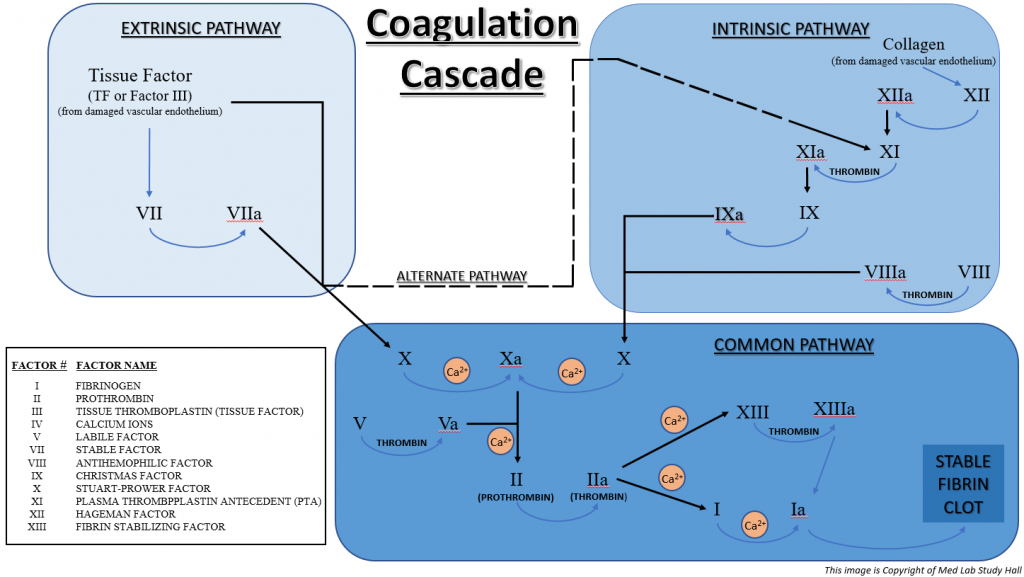
>> Stable Fibrin Clot
The end result of secondary hemostasis is the conversion of the platelet plug (formed during primary hemostasis) to a stable fibrin clot.
In Summary
Formation of a stable fibrin clot at the site of blood vessel injury requires a quite complex series of events. The injury itself triggers the processes involved in primary hemostasis (vasoconstriction, platelet adhesion, platelet activation, and platelet aggregation) to form a temporary platelet plug, as well as the clotting factors required for the elaborate coagulation cascade in secondary hemostasis that secures a final, stable fibrin clot at the site of injury.
In the weeks ahead, we will continue with our three-part series on hemostasis.
One Reply to “Hemostasis – Part 1 – Primary and Secondary Hemostasis”