Novel Virus, Old Therapy
COVID-19 (Coronavirus Disease 2019) is caused by a virus nonexistent prior to 2019, SARS-CoV-2. This means people’s immune systems, which produce protective antibodies that would destroy the virus once in their system, have no ability to quickly identify the virus as harmful and clean it out of their system.
This also means scientists do not have a vaccine available because you need to create the vaccine from the virus itself. Being a new virus, scientists had nothing to use to create a vaccine.
It takes time to grow the virus and develop the biological compounds needed to be part of the vaccine. This typically takes about 18 months or longer.
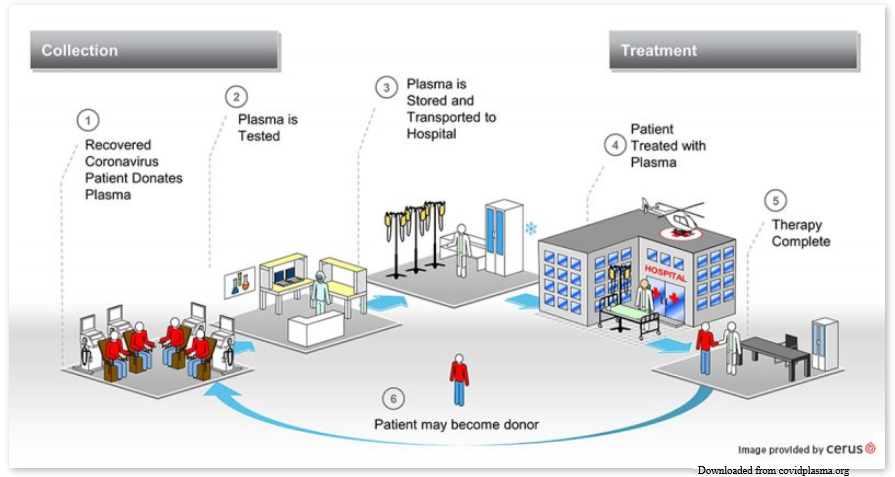
In the meantime, how should ill COVID-19 patients be treated to try to eliminate the virus? That’s when it might be beneficial to use a therapy developed in the 1890’s: convalescent plasma. http://covidplasma.org
Convalescent Plasma and Passive Immunity
What is convalescent plasma? Let’s start with the definition of plasma.
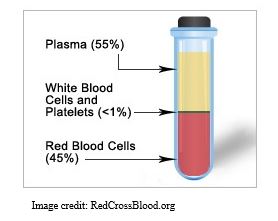
Your blood is separated into 3 primary components: red blood cells, white blood cells, and plasma. Plasma is the liquid portion of your blood left over after the red cells and white cells are removed. Convalescent means that the person has recovered from an illness and probably created antibodies to the illness-causing agent in their blood. Antibodies are protective proteins produced by the white blood cells after they are exposed to a virus or other infectious agent. These protective antibodies float freely in the plasma and when the infectious agent enters the blood, they coat it so that the immune system can remove it.
Convalescent plasma, donated by a person who recovered from an infectious disease, is then transfused to someone who is sick with that same disease. In the case of COVID-19, someone who had COVID-19 recovers and donates their plasma at a donor center. The donor center then makes that convalescent plasma available for doctors and hospitals to request for gravely ill COVID-19 patients. These patients must meet certain criteria ( set by special protocols) for eligibility to receive the plasma. If these criteria are met, the doctor and hospital will get the donated plasma from the donor center and transfuse it to the ill patent.
The antibodies against COVID-19 virus in the donated convalescent plasma enter the ill patient’s blood during the transfusion. Any COVID-19 virus present in the ill patient is coated by the donated antibodies, providing immunity to the ill patient. This type of immunity is called passive immunity because the ill patient did not produce the antibodies on their own; they were provided by another means.
Does Convalescent Plasma Work?
The verdict is still out as to the actual success of treatment with COVIID-19 convalescent plasma. Data is still being gathered from studies in multiple locations for determination of whether COVID-19 convalescent plasma helps decrease the symptoms and speeds up the recovery of severely ill COVID-19 patients.
The first randomized study with goals of determining if illness severity can be decreased and how quickly that improvement occurs is cited in an editorial in the Journal of the American Medical Association at http://jamanetwork.com.
The study showed improvement of illness severity in more than 91% of the patients who received convalescent plasma and their days to improvement decreased by nearly 5 days. Investigation must continue, as this particular study ended early and they were not able to enroll new patients due to efforts being taken to control the spread of the coronavirus. Although the data from this study are not statistically significant from a research perspective, there appears to be the potential for improvement in severely ill patients who receive COVID-19 convalescent plasma.
Other studies have been shared online prior to publication and peer review, and also support the use of COVID-19 convalescent plasma for treating severely ill COVID patients. http://pnas.org
Research Continues
Mayo Clinic (https://www.mayoclinic.org/coronavirus-covid-19) has a convalescent plasma program operating under an Investigation New Drug (IND) application with the Food and Drug Administration (FDA). COVID-19 convalescent plasma is not an FDA approved blood product. The IND allows Mayo and other organizations (such as the American Red Cross) to collect and distribute convalescent plasma for this type of treatment.
Physicians must work through the IND at Mayo to register their patients in the study and obtain products through one of the donor collection organizations working with Mayo. This allows clinical information regarding the patient to be shared for research purposes with Mayo’s research team. The patient data is deidentified prior to publication to protect each patient’s privacy.
The hope is that Mayo’s efforts will ensure that COVID-19 convalescent plasma will be available to severely ill patients. This should decrease mortality rates in that patient population and improve patient outcomes until other treatments or vaccines can be developed that are safe and effective against the SARS-CoV-2 virus.
Donation – Roll Up Your Sleeve!
If you have recovered from COVID-19 yourself and would like to help others recover from the disease, information for donating can be found at https://www.redcrossblood.org/faq.html#donating-blood-covid-19-convalescent-plasma.