Introduction
It’s 20 minutes before the end of your shift and you’re really looking forward to going home for the day. You look up from your microscope and see that a specimen from the Emergency Department has arrived. As you prepare to run it on your automated hematology analyzer for a complete blood count, the analyzer starts alarming with an error code that requires a service call.
You decide to stay and help your lab colleagues coming in for the next shift to get the Emergency Department specimen resulted out as quickly as possible. You’ll need to do a manual cell count on a hemacytometer for the white blood cell count, the red blood cell count, and the platelet count. One of your colleagues will work on the manual differential slide. It’s been a while since you’ve needed to do manual counts because the analyzer is usually very reliable, so you open up the procedure manual and follow the directions.
Hemacytometer
The laboratory device used to perform manual cell counts on a microscope is a specialized slide called a hemacytometer. It takes skill and excellent pipetting technique to achieve an accurate result. There are 2 chambers on the hemacytometer and both sides must be counted and averaged to obtain the final result.

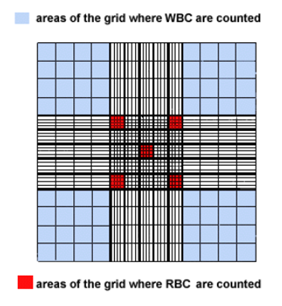
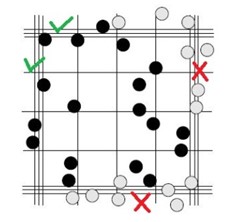
Each side has a grid etched onto the metal surface of the chamber, and the Neubauer hemacytometer grid looks like the one in this image.
The 4 corners of the grid shaded in light blue are used to count the white blood cells, and the small squares in the center shaded red are used to count the red blood cells. The entire grid is 3mm x 3mm. Therefore, each blue shaded area is 1mm x 1mm or 1mm2. The center square mm contains 25 small squares, 5 of which are used to count red blood cells. If you divide 1mm by 25, the area of the red squares is 0.04 mm2. Platelets are counted in all 25 of the small squares in the center large square (equivalent to 25 red squares). This area is 1 mm2.
The area being counted will be important for the final calculation of the cell count.
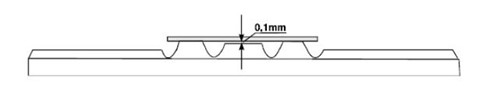
A special cover glass is needed to place on top of the metal surface, as well. This cover glass is manufactured to a precise specification that ensures the depth of the chamber between the cover glass and the metal surface is 0.1mm. This dimension will also be important in the final calculation of the cell count.
Reagents
Specimens must be diluted with an appropriate dilution fluid prior to charging the hemacytometer counting chambers. The table shows the type of cell being counted, the appropriate diluent, and the typical dilution factor used for manual counts of these cell types. Visit the Med Lab Study Hall blog Performing Dilutions for Laboratory Analysis for more information on how to perform dilutions.
| Cell type | Diluent | Dilution |
| White blood cells | Acetic Acid or Oxalate Buffer | 1:20 |
| Red blood cells | Normal Saline | 1:200 |
| Platelets | Acetic Acid or Oxalate Buffer | 1:100 |
Commercial products are available to perform each of these dilutions, such as Leuko-TIC and Thrombo-TIC systems. The advantages of using commercial diluting systems are consistency in reagents and use of premeasured volumes for dilutions, which decrease the overall error because of the manual steps involved. Many laboratories choose to prepare their own dilution fluids and perform manual dilutions, which saves money, although it slightly increases the amount of error that could be introduced if the scientist’s technique is not perfect.
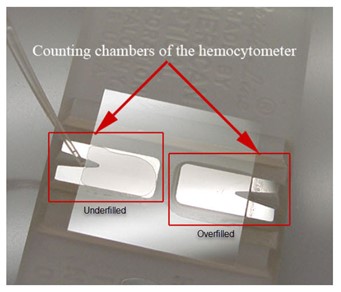
Do not overfill or underfill the hemacytometer chambers. Even the smallest deviation from optimum volume will alter the number of cells in the reading area and significantly alter the total cell count. This image shows examples of underfilled and overfilled chambers.
When this happens, you must clean the hemacytometer and coverslip using 70% alcohol, allow them to air dry, then start the chamber charging process from the beginning. A video demonstrating proper hemacytometer charging technique is available at https://youtu.be/tKu-5DMeBV0, focus on 1:00-2:00 minute marks.
Counting Technique
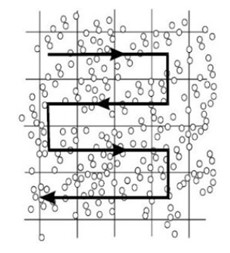
You need to remember 2 things when counting on a hemacytometer to keep yourself from counting cells more than once.
- If a cell lands on one of the grid lines, you don’t always count it when you are in that square. Choose 2 grid lines to count as you move through the grid, either the upper line and the left line, or the lower line and the right line. In the example below, this image represents one of the red squares in the center of the full grid. The scientist chose to count the cells on the upper and left grid lines, which are triple lines indicating the boundaries for the red square so that you can determine that under the microscope. There are 18 cells included in the tally for this square.
- Do not count a square more than once. Follow the same pathway across the grid every time you do a hemacytometer count. Start in the upper left and move right until you get to the end of the grid you are counting, then move down one row and move to the left until you get to the end of the grid, and continue in this fashion for the square being counted.
You can also start at the lower left and work your way up, or the right sides and work your way up or down, depending on your preference. Just be consistent each time you do a hemacytometer count so that it becomes second nature for you.
The use of a tally counter is critical so that you don’t lose your place while counting if you get interrupted.
Calculations
The basic formula for calculating final cell counts on a hemacytometer is:
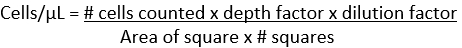
# cells counted = average counted on both sides of the hemacytometer
Depth factor = 10 (the reciprocal of 0.1 mm, which is the space between the coverslip and the hemacytometer surface)
Dilution factor = reciprocal of the dilution (if dilution is 1:200, then the factor is 200)
Area of square = length x width of one square being counted (RBC = 0.04 mm2, WBC = 1 mm2, Platelet = 1 mm2)
# squares counted = total number of squares counted on one side of the hemacytometer (RBC = 5, WBC = 4, Platelets = 1).
Example calculations for WBC and RBC
- If you counted white cells
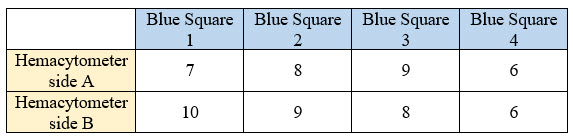
Your total count on side A = 30 and on side B = 33, then your average count would be (30+33)/2 = 31.5.
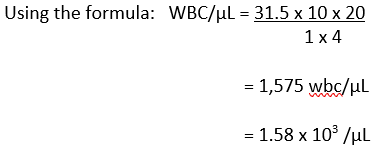
- If you counted red cells
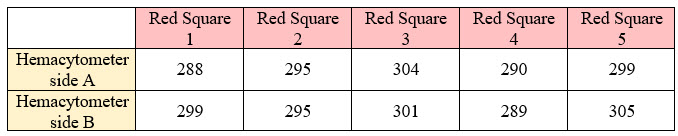
Your total count on side A = 1476 and on side B = 1489, then your average count would be (1476+1489)/2 = 1482.
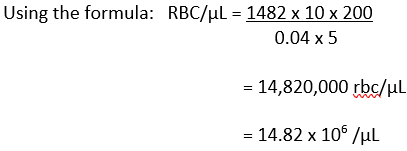
Conclusion
Backup methods of performing cell counts are essential to ensure uninterrupted patient care if analyzers happen to be out of service. These manual counts, although labor-intensive with more opportunities for error, allow patient care providers to continue evaluating and treating patients with timely, accurate lab results.
Thankfully, your skill as a medical laboratory scientist allowed you to meet the challenge!