Introduction
Did you ever wonder what blood is made of? Well, it’s more than just red-colored liquid!
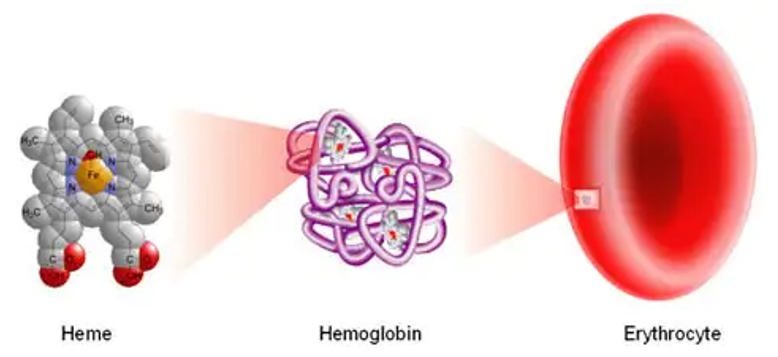
In this blog series about blood, we’ll start at the beginning with the formation of heme, an iron-containing molecule necessary for transporting oxygen and carbon dioxide in the blood.
Heme is needed to form hemoglobin. Hemoglobin is the substance in red blood cells that transports oxygen from the lungs to the tissues and transports carbon dioxide from the tissues to the lungs to be exhaled.
Red blood cells are the most abundant cellular component of your blood. They are able to flex and bend in tissue capillaries to allow oxygen delivery to every part of your body. Red blood cells are what gives your blood the red color.
Heme Formation from Precursors
There is a chain of enzymatic reactions that occurs to create heme from glycine and succinyl coenzyme A:
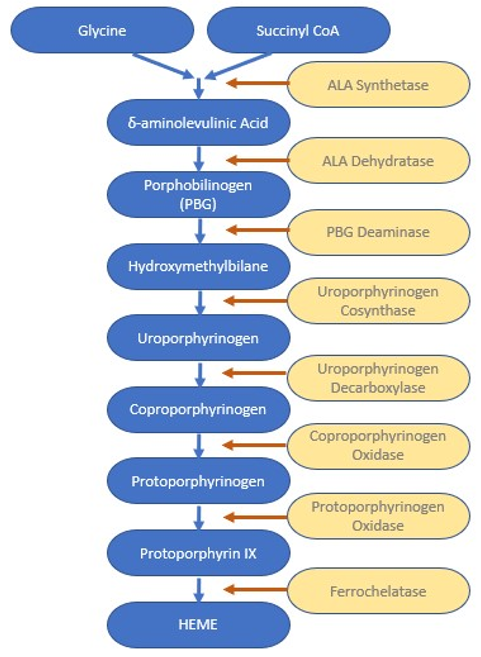
Near the end of this enzymatic sequence, protoporphyrin IX is formed, consisting of 4 pyrrole rings. The pyrrole rings are the pentagon shapes at the corners of this square representation of heme.
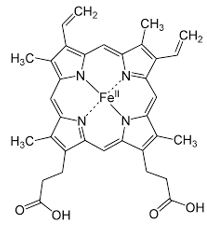
The final enzymatic step involves ferrochelatase inserting an iron atom into the center of the molecule. The iron is in the ferrous (Fe 2+) state at this point and now the molecule is called heme.
Heme for Hemoglobin
It takes 4 heme molecules to form normal hemoglobin. The heme molecules are each in the center of globin chains, which are proteins. In normal hemoglobin, there are 2 alpha-globin chains and 2 beta globin chains. Heme and globin together form hemoglobin!
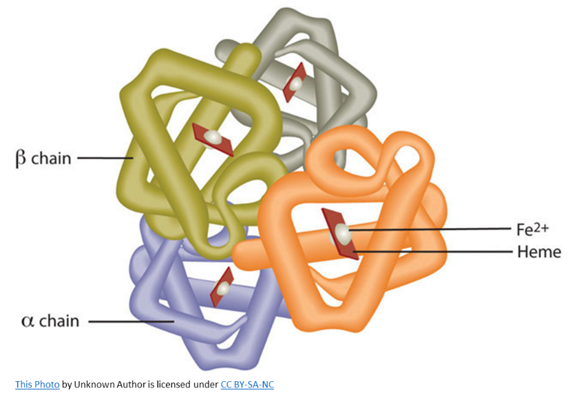
Hemoglobin can load oxygen into its structure when it encounters high tissue oxygen levels, such as in the lungs, and is referred to as oxyhemoglobin.
As the hemoglobin travels throughout the bloodstream into the rest of the body’s tissues, it encounters low tissue oxygen levels which encourages the hemoglobin to release the oxygen to the tissues and take up the carbon dioxide waste into its structure. Hemoglobin containing carbon dioxide is referred to as deoxyhemoglobin.
The deoxyhemoglobin travels in the red blood cells through the bloodstream back to the lungs, where it releases carbon dioxide and takes up more oxygen to take another trip back to the tissues.
The red blood cells’ primary purpose is transporting oxygen to the tissues and carbon dioxide to the lungs. Mature red blood cells have no nucleus and therefore cannot undergo mitosis to multiply. After about 4 months, red blood cells die and are removed from the bloodstream by the spleen.
Heme Metabolism
When red blood cells are removed from your bloodstream, the hemoglobin is recycled to create fresh hemoglobin for new red blood cells.
The globin chains are degraded into amino acids and return to the amino acid pool to make new proteins when needed.
The heme molecule is degraded into porphyrins and iron. The iron is transported to the liver for storage as ferritin until needed to form new heme molecules.
The porphyrins degrade further into bilirubin, which is transported to the liver to be conjugated with glucuronic acid and further metabolized to be excreted in the stool and urine.
Increased iron or bilirubin in your system is toxic and can lead to disease states such as iron overload or jaundice.
Conclusion
The formation of heme is a complex enzymatic process, but necessary for proper hemoglobin development. Hemoglobin’s oxygen carrying capacity is vital for your survival. Likewise, the body’s ability to degrade hemoglobin and heme after red cells die maintains a nontoxic environment in your tissues and allows specific components to be recycled for use in making new heme and proteins.
Next Blog in the Blood Blog Series – Hemoglobin
Our next blood blog will focus on the different forms of hemoglobin, including normal hemoglobins and those that cause disease. Be sure to check it out!